Pfizer vaccine authorized for children ages 12-15 by FDA
Since the approval of the Pfizer-BioNTech vaccine for 12-15 year olds, many students are receiving their first dose, even at drive-in clinics.
Middle school students and underclassmen can now receive their first dose of the Pfizer-BioNTech COVID-19 vaccine after it was approved by the Food and Drug Administration (FDA) for ages 12-15 on May 10.
According to the New York Times, about 17 million children between the ages of 12-15 are now eligible for the vaccine, which has fostered hope for a more “normal” future.
“I was excited and relieved to finally be able to get my shot,” Julia Weber ’23 said.
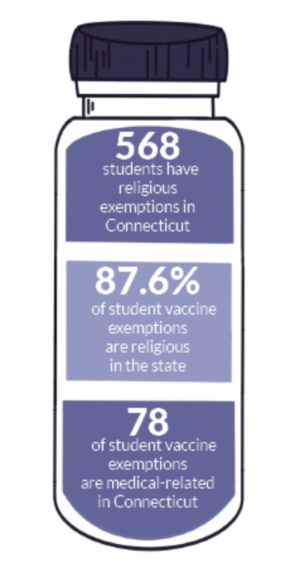
Since the authorization of the Pfizer vaccine distribution in Connecticut, the vaccine has been opened up to roughly 170,000 children, according to Westport News.
Although many students were excited to acquire their first dose, there are some who have gotten the vaccine that have experienced uncomfortable symptoms.
“I had COVID before I got my [first] vaccine and I felt worse than my friends did when they got theirs,” Bea Hobbs ’23 said. “I felt really sick and tired.”
Though the idea of returning to a normal and COVID-free life after the approval of the vaccine has thrilled many students, others believe we should continue to stay cautious.
“We should be keeping in mind the kids that are too young to get it and the people who have health conditions and can’t get [the vaccine],” Weber said.
The rollout of the Pfizer vaccine for the middle and high school students has created a more hopeful outlook towards the future as more individuals become fully vaccinated, despite the fact that masks are still required in schools.
“After this long journey, it looks like almost everything is coming back together,” Juliet Tracey ’23 said. “[It looks like] society as a whole is picking back up to where we left off.”